Contents
Planck’s Quantum Theory of Radiation
By the end of 19th century, although Maxwell’s theory of Electromagnetic wave successfully explained the phenomena like interference, diffraction, polarization etc., it failed to explain various other phenomena like photoelectric effect, distribution of energy in the spectrum of black body radiation, presence of spectral lines in the spectrum of hydrogen of atom etc.
Further, although Wien, Rayleigh and Jeans were partially successful in explaining the energy distribution in black body radiation there was a significant discrepancy between the predictions from the classical theory and the experimentally observed spectral emissivity curves of black body radiation. To reconcile this discrepancy, Planck put forward his Quantum Theory of Radiation. The quantum theory of radiation becomes widely accepted only when Einstein used it to explain Photoelectric effect.
According to Planck’s quantum theory of radiation, the radiations come out of a source and also interact with matter in the form of discrete energy packets, called ‘photons’ or ‘quanta’. Each photon contains a specific amount of energy depending upon the wavelength (frequency) of radiation.
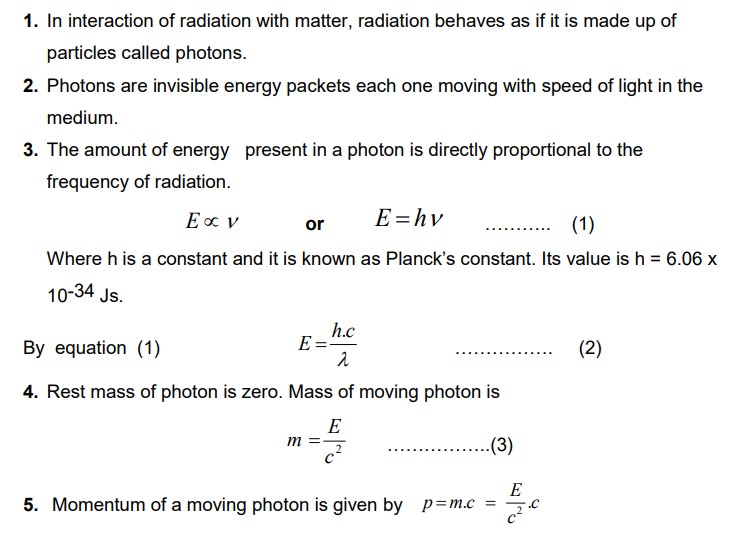
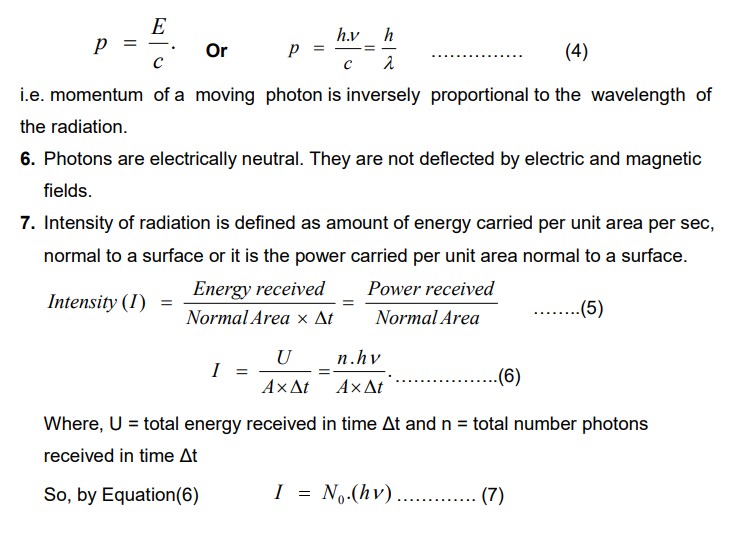
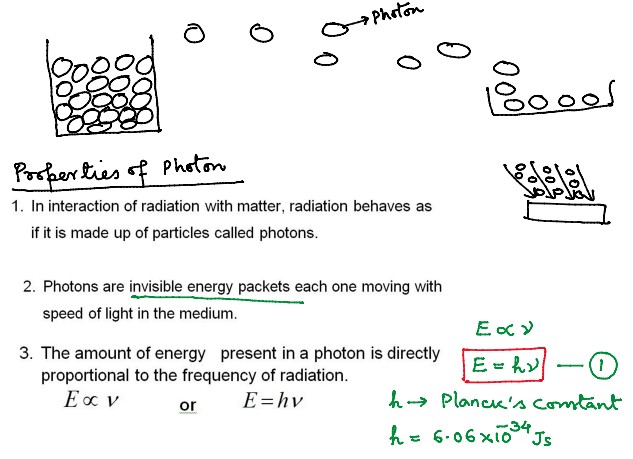
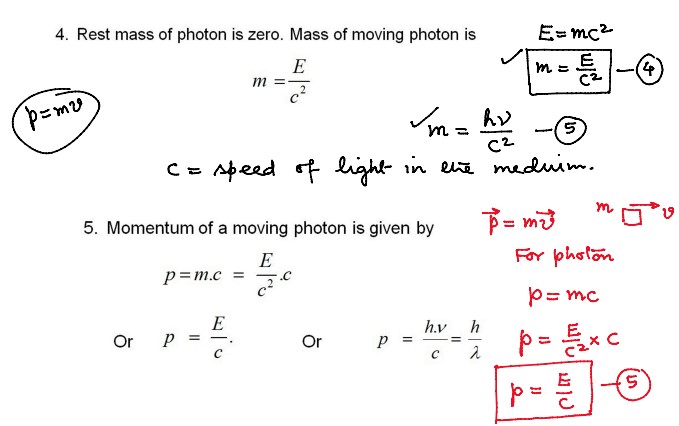
Properties of Photons

Lecture Video[In Hindi+English Language]
Lecture Notes
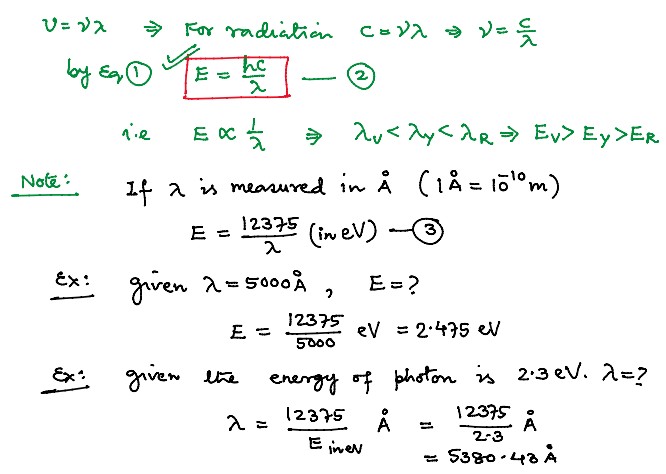
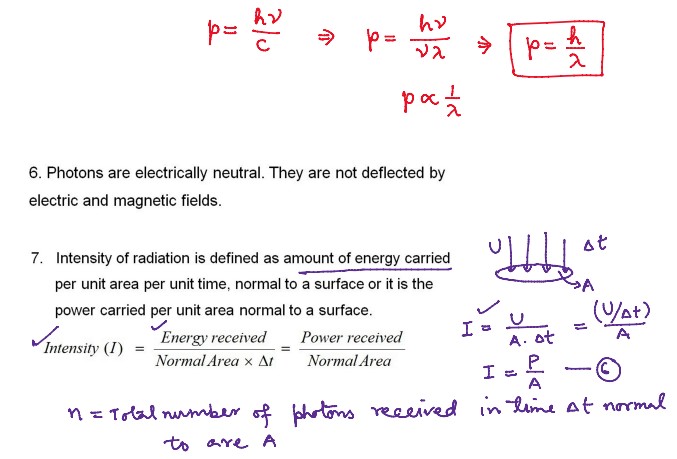

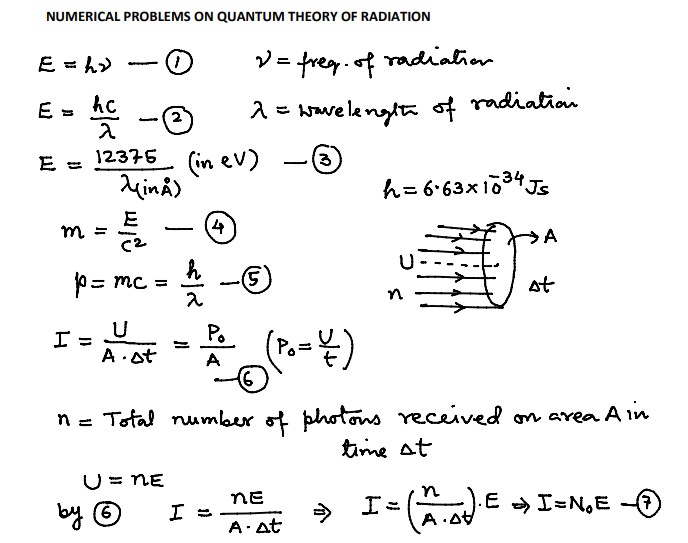
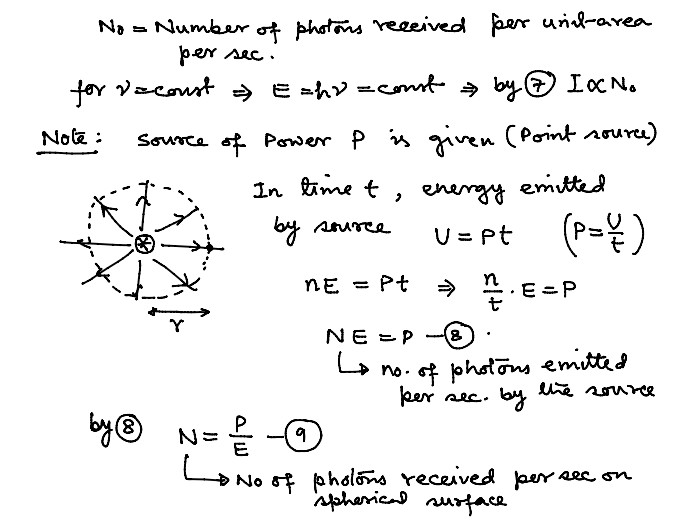
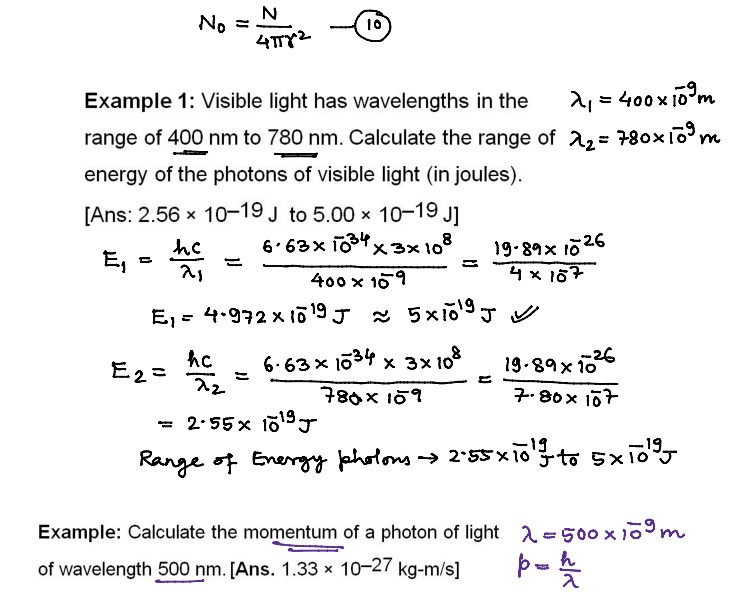
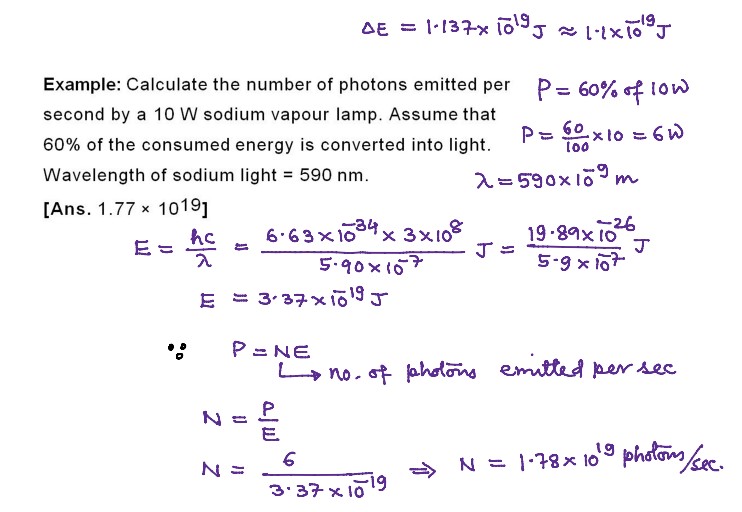
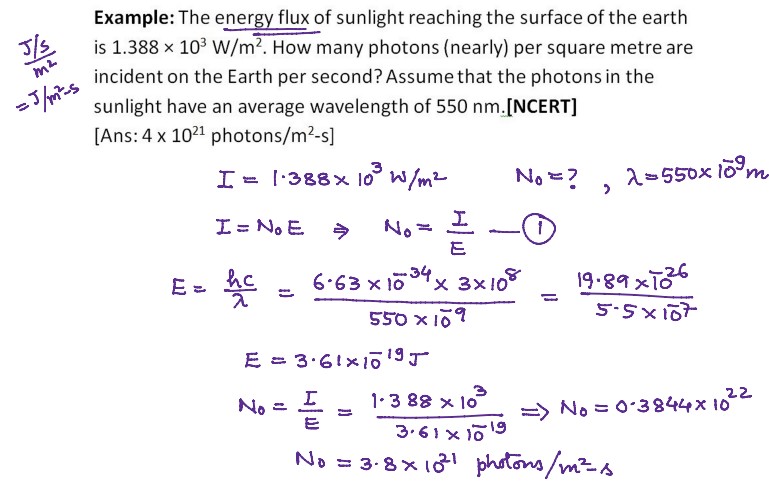
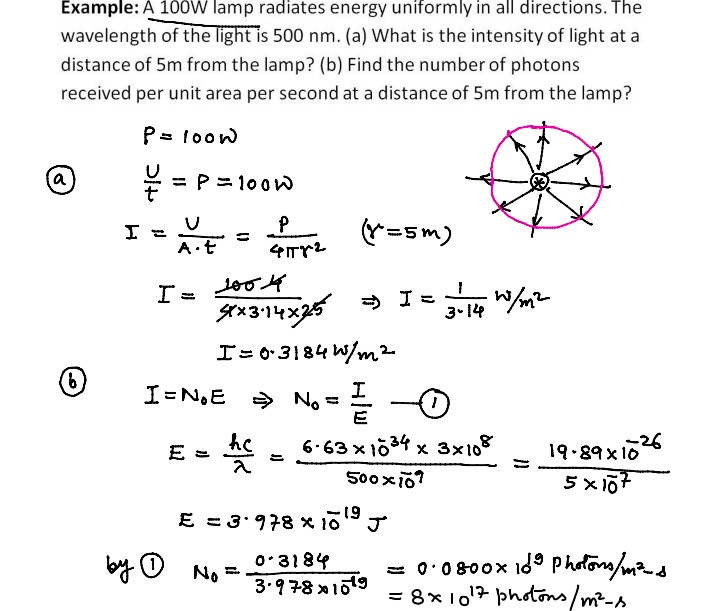
Numerical Problems on Quantum Theory of Radiation
Lecture Video[In Hindi+English Language]
Lecture Notes (Numerical Problems)